3,9-Diethylidene-2,4,8,10-tetraoxaspiro(5.5)undecane

| |
| Names | |
|---|---|
| Preferred IUPAC name
3,9-Diethylidene-2,4,8,10-tetraoxaspiro[5.5]undecane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.254.405 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C11H16O4 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
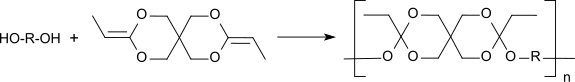
3,9-Diethylidene-2,4,8,10-tetraoxaspiro[5.5]undecane (DETOSU) is a bicyclic ketene acetal derived from the isomeric allyl acetal 3,9-divinyl-2,4,8,10-tetraoxaspiro[5.5]undecane (DVTOSU). As a bifunctional monomer, DETOSU is an important building block for polyorthoesters formed by the addition of diols to the activated double bond of the diketene acetal.[1]
Preparation[edit]
DETOSU is prepared in a rearrangement reaction of DVTOSU, it is an exothermic reaction that also occurs spontaneously with complete conversion.[2] For production on an industrial scale, the rearranged is carried out at elevated temperatures in the presence of catalysts.
The rearrangement reaction can be carried out in alkaline medium (such as with n-butyllithium in ethylenediamine[3] or potassium tert-butoxide in ethylenediamine[4]) but also photochemically by UV irradiation in the presence of iron pentacarbonyl as catalyst and triethylamine in boiling pentane[5] or with dichlorotris(triphenylphosphine)ruthenium(II) / sodium carbonate in bulk.[6][7]
In order to obtain purities sufficient for the use as a monomer, the crude product (obtained after the rearrangement reaction and vacuum distillation) must be recrystallized several times from pentane. The yields of pure product are about 50%.[3]
Properties[edit]
3,9-Diethylidene-2,4,8,10-tetraoxaspiro[5.5]undecane is, when pure, a crystalline material at room temperature.[3] Because of its low crystallization tendency, it is mostly used as a liquid. DETOSU is relatively unstable. It hydrolyzes rapidly even in the presence of only water traces and spontaneously isomerizes during storage to the diallylacetal DVTOSU, which is inactive for the polymerization.[8] The pure substance is very reactive against the attack of electrophilic agents and ows a strong tendency for cationic polymerization.[6] A characteristic property of DETOSU is the intense IR band at 1700 cm−1, which is also used to monitor the conversion in the rearrangement reaction.
Use[edit]
The diketene acetal 3,9-diethylidene-2,4,8,10-tetraoxaspiro[5.5]undecane, DETOSU, is a reactive bifunctional monomer that forms biodegradable polyorthoesters by polyaddition with α,ω-diols.
Polyorthoesters are used as embedding media for pharmaceuticals in extended release formulations for controlled drug release by surface erosion under physiological conditions.[9]
References[edit]
- ^ Heller, J.; Himmelstein, K. J. (1985). "Poly(ortho ester) biodegradable polymer systems". Drug and Enzyme Targeting. Methods in Enzymology. Vol. 112. pp. 422–436. doi:10.1016/S0076-6879(85)12033-1. ISBN 9780121820121. PMID 3930918.
- ^ Pişkin, E. (2002). "Biodegradable Polymers in Medicine". In Scott, Gerald (ed.). Degradable Polymers (2nd ed.). Kluwer Academic Press. pp. 321–378. doi:10.1007/978-94-017-1217-0_10. ISBN 1-4020-0790-6.
- ^ a b c US 5939453, J. Heller, S.Y. Ng, "PEG-POE, PEG-POE-PEG, and POE-PEG-POE block copolymers", published 1999-8-17, assigned to Advanced Polymer Systems, Inc.
- ^ US 4532335, R.F. Helwing, "Preparation of ketene acetals by rearrangement of allyl and substituted allyl acetals", published 1985-07-30, assigned to SRI International
- ^ US 6863782, P.W. Newsome et al., "Method of preparing di(ketene acetals)", published 2005-3-8, assigned to A.P. Pharma, Inc.
- ^ a b Crivello, J. V.; Malik, R.; Lai, Y.-L. (1996). "Ketene acetal monomers: Synthesis and characterization". J. Polym. Sci. A Polym. Chem. 34 (15): 3091–3102. Bibcode:1996JPoSA..34.3091C. doi:10.1002/(SICI)1099-0518(19961115)34:15<3091::AID-POLA1>3.0.CO;2-0.
- ^ Heller, Jorge (2011). "Poly(Ortho Esters)". In Lendlein, Andreas; Sisson, Adam (eds.). Handbook of Biodegradable Polymers: Isolation, Synthesis, Characterization and Applications. Wiley-VCH. pp. 77–105. doi:10.1002/9783527635818.ch4. ISBN 978-3-527-32441-5.
- ^ Heller, Jorge (1993). "Poly(Ortho Esters)". In Langer, Robert S.; Peppas, Nicholas A. (eds.). Biopolymers I. Advances in Polymer Science. Berlin / Heidelberg: Springer-Verlag. pp. 41–92. ISBN 3-540-56148-X.
- ^ Heller, Jorge (1990). "Development of poly(ortho esters): A historical overview". Biomaterials. 11 (9): 659–665. doi:10.1016/0142-9612(90)90024-K. PMID 2090300.