Cyclic alkyl amino carbenes
In chemistry, cyclic(alkyl)(amino)carbenes (CAACs) are a family of stable singlet carbene ligands developed by the research group of Guy Bertrand in 2005 at UC Riverside.[1] In marked contrast with the popular N-heterocyclic carbenes (NHCs) which possess two "amino" substituents adjacent to the carbene center, CAACs possess one "amino" substituent and an sp3 carbon atom "alkyl". This specific configuration makes the CAACs very good σ-donors (higher HOMO) and π-acceptors (lower LUMO) when compared to NHCs. Moreover the reduced heteroatom stabilization of the carbene center in CAACs versus NHCs also gives rise to a smaller ΔEST (48.3 vs 72.7 kcal mol-1).
Synthesis[edit]
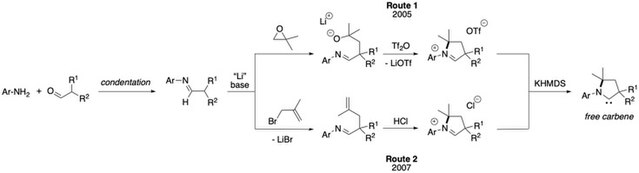
The original preparation of CAACs precursors (Route 1)[1] begins with condensation of 2,6-diisopropylaniline and 2-methylpropanal. Deprotonation of this aldimine with lithium diisopropylamide gives an aza-allyl anion, which ring opens 1,2-epoxy-2-methylpro-pane. The resulting lithium alkoxide is then treated with triflic anhydride to generate the aldiminium salt. Another methods (Route 2) involves alkylation of the aldimine with 3-bromo-2-methylpropene to generate an alkenyl aldimine, which cyclises to the corresponding iminium salts in the presence of HCl upon heating.[2],[3],[4] This straightforward approach allows for kilogram-scale syntheses of CAAC precursors. Finally, deprotonation of the iminimum salts with potassium bis(trimethylsilyl)amide affords the free carbene as a white solid. CAAC free carbenes are air and moisture sensitive but can be stored for weeks under an inert atmosphere.
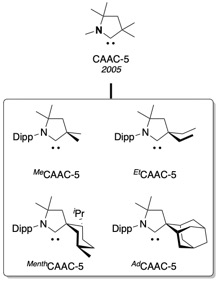
Family of CAAC ligands[edit]

Since 2005, the family of cyclic (alkyl)(amino)carbenes expended to encompass the functionalized FunCAACs,[5] the BiCAACs with a bicyclic backbone,[6] the CAAC-6s with a 6-membered backbone,[7] and the chiral ChiCAACs used in asymmetric catalysis.[8]
Reactions[edit]
Cyclic (alkyl)(amino)carbenes have found to "stabilize" (for adducts of) highly reactive species.[9],[10] Better σ-donors and π-acceptors than the well-known N-heterocyclic carbenes (NHCs), these stable singlet carbene are well known for stabilising highly reactive species, such as highly reactive low valent complexes,[11] and main group radicals.[12][13]

As ligand for transition metal catalysts, CAAC-Ru complexes catalyze ethenolysis.[14] Note that this was the first time ruthenium metathesis catalysts exhibited high performance in cross‐metathesis reactions employing ethylene gas, with activities sufficient for the industrial‐scale production of linear α‐olefins (LAOs) and other terminal‐olefin products.
CAACs are components of LEDs.[15][16][17]
It was also demonstrated that their ambiphilic nature allows them to participate in the activation of enthalpically strong E-H bonds (E: N, P, Si, …),[18] a distinctive feature traditionally reserved to transition metals. It was also shown that bulky CAACs promote the reverse transformation,[19] a formal reductive elimination of E-H bonds at carbon, further delineating the parallel with transition metals.
References[edit]
- ^ a b Lavallo, Vincent; Canac, Yves; Präsang, Carsten; Donnadieu, Bruno; Bertrand, Guy (2005-09-05). "Stable Cyclic (Alkyl)(Amino)Carbenes as Rigid or Flexible, Bulky, Electron-Rich Ligands for Transition-Metal Catalysts: A Quaternary Carbon Atom Makes the Difference". Angewandte Chemie International Edition. 44 (35): 5705–5709. doi:10.1002/anie.200501841. ISSN 1521-3773. PMC 2427276. PMID 16059961.
- ^ Müller, Carsten; Andrada, Diego M.; Bischoff, Inga-Alexandra; Zimmer, Michael; Huch, Volker; Steinbrück, Nils; Schäfer, André (2019-03-11). "Synthesis, Structure, and Bonding Analysis of Tin(II) Dihalide and Cyclopentadienyltin(II) Halide (Alkyl)(amino)carbene Complexes". Organometallics. 38 (5): 1052–1061. doi:10.1021/acs.organomet.8b00861. ISSN 0276-7333. S2CID 104422050.
- ^ Jazzar, Rodolphe; Dewhurst, Rian D.; Bourg, Jean-Baptiste; Donnadieu, Bruno; Canac, Yves; Bertrand, Guy (2007-04-13). "Intramolecular 'Hydroiminiumation' of Alkenes: Application to the Synthesis of Conjugate Acids of Cyclic Alkyl Amino Carbenes (CAACs)". Angewandte Chemie International Edition. 46 (16): 2899–2902. doi:10.1002/anie.200605083. PMC 2440680. PMID 17352445.
- ^ Jazzar, Rodolphe; Bourg, Jean-Baptiste; Dewhurst, Rian D.; Donnadieu, Bruno; Bertrand, Guy (April 2007). "Intramolecular 'Hydroiminiumation and -amidiniumation' of Alkenes: A Convenient, Flexible, and Scalable Route to Cyclic Iminium and Imidazolinium Salts". The Journal of Organic Chemistry. 72 (9): 3492–3499. doi:10.1021/jo0703909. ISSN 0022-3263. PMC 2440693. PMID 17408289.
- ^ Chu, Jiaxiang; Munz, Dominik; Jazzar, Rodolphe; Melaimi, Mohand; Bertrand, Guy (2016-06-29). "Synthesis of Hemilabile Cyclic (Alkyl)(amino)carbenes (CAACs) and Applications in Organometallic Chemistry". Journal of the American Chemical Society. 138 (25): 7884–7887. doi:10.1021/jacs.6b05221. ISSN 0002-7863. PMID 27304485.
- ^ Tomás-Mendivil, Eder; Hansmann, Max M.; Weinstein, Cory M.; Jazzar, Rodolphe; Melaimi, Mohand; Bertrand, Guy (2017-06-14). "Bicyclic (Alkyl)(amino)carbenes (BICAACs): Stable Carbenes More Ambiphilic than CAACs". Journal of the American Chemical Society. 139 (23): 7753–7756. doi:10.1021/jacs.7b04640. ISSN 0002-7863. PMID 28541687.
- ^ Weinstein, Cory M.; Junor, Glen P.; Tolentino, Daniel R.; Jazzar, Rodolphe; Melaimi, Mohand; Bertrand, Guy (2018-07-25). "Highly Ambiphilic Room Temperature Stable Six-Membered Cyclic (Alkyl)(amino)carbenes". Journal of the American Chemical Society. 140 (29): 9255–9260. doi:10.1021/jacs.8b05518. ISSN 0002-7863. PMID 29954178. S2CID 207192160.
- ^ Pichon, Delphine; Soleilhavoup, Michele; Morvan, Jennifer; Junor, Glen P.; Vives, Thomas; Crévisy, Christophe; Lavallo, Vincent; Campagne, Jean-Marc; Mauduit, Marc; Jazzar, Rodolphe; Bertrand, Guy (2019). "The debut of chiral cyclic (alkyl)(amino)carbenes (CAACs) in enantioselective catalysis". Chemical Science. 10 (33): 7807–7811. doi:10.1039/C9SC02810B. ISSN 2041-6520. PMC 6761915. PMID 31588330.
- ^ Soleilhavoup, Michèle; Bertrand, Guy (2015-02-17). "Cyclic (Alkyl)(Amino)Carbenes (CAACs): Stable Carbenes on the Rise". Accounts of Chemical Research. 48 (2): 256–266. doi:10.1021/ar5003494. ISSN 0001-4842. PMID 25515548.
- ^ Melaimi, Mohand; Jazzar, Rodolphe; Soleilhavoup, Michèle; Bertrand, Guy (2017). "Cyclic (Alkyl)(amino)carbenes (CAACs): Recent Developments". Angewandte Chemie International Edition. 56 (34): 10046–10068. doi:10.1002/anie.201702148. ISSN 1521-3773. PMID 28376253.
- ^ Roy, Sudipta; Mondal, Kartik Chandra; Roesky, Herbert W. (2016-03-15). "Cyclic Alkyl(amino) Carbene Stabilized Complexes with Low Coordinate Metals of Enduring Nature". Accounts of Chemical Research. 49 (3): 357–369. doi:10.1021/acs.accounts.5b00381. ISSN 0001-4842. PMID 26925983.
- ^ Kundu, Subrata; Sinhababu, Soumen; Chandrasekhar, Vadapalli; Roesky, Herbert W. (2019-05-08). "Stable cyclic (alkyl)(amino)carbene (cAAC) radicals with main group substituents". Chemical Science. 10 (18): 4727–4741. doi:10.1039/C9SC01351B. ISSN 2041-6539. PMC 6510188. PMID 31160949.
- ^ Ullrich, Tobias; Pinter, Piermaria; Messelberger, Julian; Haines, Philipp; Kaur, Ramandeep; Hansmann, Max M.; Munz, Dominik; Guldi, Dirk M. (2020). "Singlet Fission in Carbene-Derived Diradicaloids". Angewandte Chemie International Edition. 59 (20): 7906–7914. doi:10.1002/anie.202001286. ISSN 1521-3773. PMC 7317569. PMID 32129920.
- ^ Marx, Vanessa M.; Sullivan, Alexandra H.; Melaimi, Mohand; Virgil, Scott C.; Keitz, Benjamin K.; Weinberger, David S.; Bertrand, Guy; Grubbs, Robert H. (2015). "Cyclic Alkyl Amino Carbene (CAAC) Ruthenium Complexes as Remarkably Active Catalysts for Ethenolysis". Angewandte Chemie International Edition. 54 (6): 1919–1923. doi:10.1002/anie.201410797. ISSN 1521-3773. PMC 4713124. PMID 25522160.
- ^ Di, Dawei; Romanov, Alexander S.; Yang, Le; Richter, Johannes M.; Rivett, Jasmine P. H.; Jones, Saul; Thomas, Tudor H.; Jalebi, Mojtaba Abdi; Friend, Richard H.; Linnolahti, Mikko; Bochmann, Manfred (2017-04-14). "High-performance light-emitting diodes based on carbene-metal-amides". Science. 356 (6334): 159–163. arXiv:1606.08868. doi:10.1126/science.aah4345. ISSN 0036-8075. PMID 28360136. S2CID 206651900.
- ^ Hamze, Rasha; Peltier, Jesse L.; Sylvinson, Daniel; Jung, Moonchul; Cardenas, Jose; Haiges, Ralf; Soleilhavoup, Michele; Jazzar, Rodolphe; Djurovich, Peter I.; Bertrand, Guy; Thompson, Mark E. (2019-02-08). "Eliminating nonradiative decay in Cu(I) emitters: >99% quantum efficiency and microsecond lifetime". Science. 363 (6427): 601–606. doi:10.1126/science.aav2865. ISSN 0036-8075. PMID 30733411. S2CID 59621722.
- ^ Jazzar, Rodolphe; Soleilhavoup, Michele; Bertrand, Guy (2020-05-13). "Cyclic (Alkyl)- and (Aryl)-(amino)carbene Coinage Metal Complexes and Their Applications". Chemical Reviews. 120 (9): 4141–4168. doi:10.1021/acs.chemrev.0c00043. ISSN 0009-2665. OSTI 1773893. PMID 32239922. S2CID 214771584.
- ^ Frey, G. D.; Lavallo, V.; Donnadieu, B.; Schoeller, W. W.; Bertrand, G. (2007-04-20). "Facile Splitting of Hydrogen and Ammonia by Nucleophilic Activation at a Single Carbon Center". Science. 316 (5823): 439–441. doi:10.1126/science.1141474. ISSN 0036-8075. PMID 17446400. S2CID 45106592.
- ^ Tolentino, Daniel R.; Neale, Samuel E.; Isaac, Connie J.; Macgregor, Stuart A.; Whittlesey, Michael K.; Jazzar, Rodolphe; Bertrand, Guy (2019-06-26). "Reductive Elimination at Carbon under Steric Control". Journal of the American Chemical Society. 141 (25): 9823–9826. doi:10.1021/jacs.9b04957. ISSN 0002-7863. PMID 31180660. S2CID 184484268.
