CXorf65
| CXorf65 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | CXorf65, chromosome X open reading frame 65 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | MGI: 2685460 HomoloGene: 52739 GeneCards: CXorf65 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Human uncharacterized protein CXorf65 is encoded by the gene CXorf65, which is located on the minus strand of chromosome X.[5] Its transcript is 834 nucleotides long and consists of 6 exons.[6] The translated protein is 183 amino acids in length.[7] with a molecular weight of 21.3 kDa[8][9]
Gene[edit]
Human chromosome X open reading frame 65 (CXorf65), also known as LOC158830[10] or A6NEN9,[11] spans 2,852 base pairs on the minus strand of chromosome X at Xq13.1.[12] It belongs to the gene family pfam 15874;[13] a two-member family for conserved putative interleukin 2 receptor gamma chain (Il2rg) domains. Additionally, human CXorf65 is one of 413 genes which belong to gene cluster 31: Spermatids – Spermatogenesis.[14]

Transcript[edit]
Human CXorf65's mRNA transcript contains 6 exons which form an 834 nucleotide strand.[6]

Isoforms[edit]
Human CXorf65 has three isoforms: uncharacterized protein CXorf65, uncharacterized protein CXorf65 isoform X1, and a non-coding RNA sequence.[5] Only uncharacterized protein CXorf65 produces a functional product.
Human uncharacterized protein CXorf65 isoform X1 is an alternative splicing that results in the exclusion of exon 4, which shortens the transcript by 69 base pairs and ultimately leads to a nonfunctional protein.[15] The non-coding RNA sequence suffers from a frameshift mutation due to a 19 bp deletion in exon 2.[16] This results in a premature stop codon 126 bp from the start of translation.
| Transcript | mRNA Accession | Nucleotides | Exons | Protein Accession | Amino Acids |
|---|---|---|---|---|---|
| Uncharacterized protein CXorf65 | NM_001025265.3 | 834 | 6 | NP_001020436.1 | 183 |
| Uncharacterized protein CXorf65 isoform X1 | XM_005262244.5 | 765 | 5 | XP_005262301.1 | 160 |
| Non-coding RNA Sequence | NR_033212.2 | 815 | 6 | Non-coding | Non-coding |
Expression[edit]
Human CXorf65 is ubiquitously expressed throughout the body at low levels, typically ranging from 1-7 RPKM in most tissues;[5] however, its expression has an affinity for testis, adrenal, thymus, and bone marrow tissues which can lead to RPKM levels increasing to 6-14 RPKM[17] Additionally, expression can spike to as high as 27 RPKM within adrenal tissues during week 20 of fetal development.[5]
Protein[edit]

Human uncharacterized protein CXorf65 consists of 183 amino acids.[7] has a molecular weight of 21.3 kDa,[8][9] and a predicted isoelectric point of 10.33.[8] CXorf65's protein product is predicted to primarily localize within the nucleus.[18]
Expression[edit]
Human CXorf65 maintains a low whole organism protein abundance at 0.062 ppm2.[19] It has also been identified as a member of the spermatozoa proteome.[20]
Regulation[edit]
Human uncharacterized protein CXorf65 has a bipartite nuclear localization signal,[21] which regulates its transport into the nucleus.
Structure[edit]
Human uncharacterized CXorf65's secondary structure is predicted to have five sections of β-sheets and four sections of α-helixesm[22] The corresponding tertiary structure is thus predicted to be a β-grasp fold[23] accompanied by a long basic (positively charged) tail.[22]

Post-Translational Modifications[edit]
Human uncharacterized protein CXorf65 has one predicted casein kinase II phosphorylation[24][25] site and two predicted acetylation sites.[25] Additionally, uncharacterized protein CXorf65 has predicted motifs for N-terminal degradation via type II destabilizing residues and a non-covalent binding site for SUMO (small ubiquitin-like modifier) proteins.[24]

Homology[edit]
Orthologs[edit]

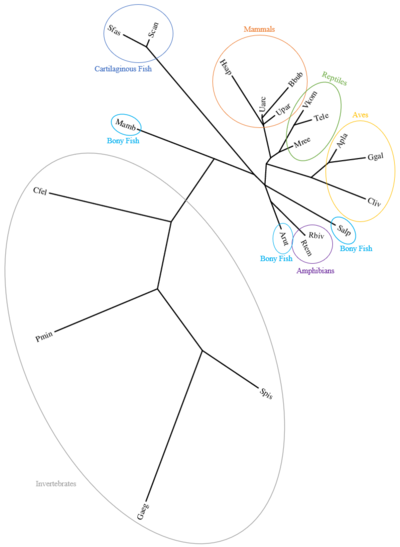
Human CXorf65 orthologs exist in mammals, reptiles, aves, amphibians, bony fish, cartilaginous fish, and the following invertebrates: Cnidaria, Platyhelminthes, Annelida, Arthropoda, Mollusca, Rotifera, Lophophorata, Echinodermata, Hemichordate Amphioxiformes, and Tunicata[26]

Paralogs[edit]
There are no human paralogs of CXorf65;[27] however, there is a paralogous Il2rg domain within C22orf15,[13]

Evolution[edit]
CXorf65 is a moderately evolving gene in reference to fibrinogen alpha and cytochrome c.[28][29]

Function[edit]
Interacting Proteins[edit]
CXorf65 has been documented to co-express with IL2RG in Mus musculus (mouse).[30] an interleukin subunit coding gene located within the same gene neighborhood in humans at Xq13.1.[31] Fusions between these two genes have been observed within the following organisms: Sarcophilus harrisii (Tasmanian devil), Felis catus (Cat), Cavia porcellus (Guinea pig), Ictidomys tridecemlineatus (Thirteen-lined ground squirrel), Rattus norvegicus (Brown rat), and Mus musculus (Mouse)[30]
Clinical Significance[edit]
Health & Disease[edit]
Differential expression of CXorf65 in humans is correlated to azoospermia and impaired spermatogenesis[32] while general expression of the gene has been linked to an improved prognosis in urothelial[33] and ovarian cancer.[10] In WG4 temporal lobe epilepsy, human CXorf65 undergoes hypermethylation.[34] In cases of disc herniation,[35] acute coronary syndrome[36] and with the presence of TGF-β in eosinophils[37] human CXorf65 is downregulated.
References[edit]
- ^ a b c GRCh38: Ensembl release 89: ENSG00000204165 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000090141 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b c d "Gene 158830". National Center for Biotechnology (NCBI). U.S. National Library of Medicine.
- ^ a b "NCBI Reference Sequence: NM_001025265.3". National Center for Biotechnology (NCBI). U.S. National Library of Medicine. 2 June 2022.
- ^ a b "Uncharacterized protein CXorf65". National Center for Biotechnology (NCBI). U.S. National Library of Medicine.
- ^ a b c "Compute pI/Mw tool". Expasy.
- ^ a b "Statistical Analysis of Protein Sequences Tool". SAPS. European Molecular Biology Laboratory (EMBL)- European Bioinformatics Institute (EBI).
- ^ a b An Y, Yang Q (2020). "Development and Validation of an Immune-Related Prognostic Signature for Ovarian Cancer Based on Weighted Gene Coexpression Network Analysis". BioMed Research International. 2020: 7594098. doi:10.1155/2020/7594098. PMC 7749778. PMID 33381581.
- ^ "A6NEN9". UniProt.
- ^ "Genome Data Viewer: NC_000023.11". National Center for Biotechnology (NCBI). U.S. National Library of Medicine.
- ^ a b "pfam15874". National Center for Biotechnology (NCBI). U.S. National Library of Medicine.
- ^ "CXorf65". Human Protein Atlas.
- ^ "Isoform X1 mRNA". National Center for Biotechnology (NCBI). U.S. National Library of Medicine. 5 April 2022.
- ^ "NCBI Reference Sequence: NR_033212.2". National Center for Biotechnology (NCBI). U.S. National Library of Medicine. 18 April 2022.
- ^ "NCBI Reference Sequence: NR_033212.2 (GDS423)". National Center for Biotechnology (NCBI). U.S. National Library of Medicine.
- ^ "PSORT II Prediction Signal-seq tool".
- ^ "CXorf65". PAXdb: Protein Abundance Database.
- ^ Vandenbrouck Y, Lane L, Carapito C, Duek P, Rondel K, Bruley C, et al. (November 2016). "Looking for Missing Proteins in the Proteome of Human Spermatozoa: An Update" (PDF). Journal of Proteome Research. 15 (11): 3998–4019. doi:10.1021/acs.jproteome.6b00400. PMID 27444420.
- ^ "Myhits". Myhits Motif Scan. Swiss Institute of Bioinformatics (SIB).
- ^ a b "Uncharacterized protein CXorf65". Alphafold.
- ^ "Job ID: c2f0c773ba05d9ac". Phyre2.
- ^ a b "ELM Functional Site Prediction tool". The Eukaryotic Linear Motif (ELM) resource.
- ^ a b "CXorf65 histone modifications". PhosphoSite.
- ^ "Basic Local Alignment Search Tool (BLAST)". National Center for Biotechnology Information (NCBI). U.S. National Library of Medicine.
- ^ "CXorf65". GeneCards.
- ^ "Divergence Time". TimeTree.
- ^ "EMBOSS Needle". European Molecular Biology Laboratory (EMBL)- European Bioinformatics Institute (EBI).
- ^ a b "CXorf65". STRING.
- ^ "IL2RG". GeneCards.
- ^ Omolaoye TS, Omolaoye VA, Kandasamy RK, Hachim MY, Du Plessis SS (February 2022). "Omics and Male Infertility: Highlighting the Application of Transcriptomic Data". Life. 12 (2): 280. Bibcode:2022Life...12..280O. doi:10.3390/life12020280. PMC 8875138. PMID 35207567.
- ^ Pagliarulo F, Cheng PF, Brugger L, van Dijk N, van den Heijden M, Levesque MP, et al. (2022). "Molecular, Immunological, and Clinical Features Associated With Lymphoid Neogenesis in Muscle Invasive Bladder Cancer". Frontiers in Immunology. 12: 793992. doi:10.3389/fimmu.2021.793992. PMC 8821902. PMID 35145509.
- ^ Miller-Delaney SF, Bryan K, Das S, McKiernan RC, Bray IM, Reynolds JP, et al. (March 2015). "Differential DNA methylation profiles of coding and non-coding genes define hippocampal sclerosis in human temporal lobe epilepsy". Brain. 138 (Pt 3): 616–631. doi:10.1093/brain/awu373. PMC 4408428. PMID 25552301.
- ^ Zheng HL, Xu WN, Chen PB, Jiang LS, Zheng XF, Jiang SD (2022). "Increased Expression of Prolyl Endopeptidase Induced by Oxidative Stress in Nucleus Pulposus Cells Aggravates Intervertebral Disc Degeneration". Oxidative Medicine and Cellular Longevity. 2022: 9731800. doi:10.1155/2022/9731800. PMC 9020979. PMID 35464773.
- ^ Li M, Ren C, Wu C, Li X, Li X, Mao W (2020). "Bioinformatics Analysis Reveals Diagnostic Markers and Vital Pathways Involved in Acute Coronary Syndrome". Cardiology Research and Practice. 2020: 3162581. doi:10.1155/2020/3162581. PMC 7670299. PMID 33224526.
- ^ Shen ZJ, Hu J, Esnault S, Dozmorov I, Malter JS (September 2015). "RNA Seq profiling reveals a novel expression pattern of TGF-β target genes in human blood eosinophils". Immunology Letters. 167 (1): 1–10. doi:10.1016/j.imlet.2015.06.012. PMC 6208316. PMID 26112417.




