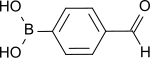
4-Formylphenylboronic acid
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
4-(Dihydroxyboranyl)benzaldehyde | |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.103.550 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C7H7BO3 | |
| Molar mass | 149.94 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
4-Formylphenylboronic acid (4-FPBA) is a versatile synthetic building block and an important intermediate in the preparation of agrochemical and pharmaceutical active ingredients. The substance finds industrial application as a stabilizer and inhibitor for enzymes[1] and as a bactericide.
Synthesis[edit]
The synthesis of 4-formylyphenylboronic acid was reported by the group of Heinrich Nöth in 1990. 4-Bromobenzaldehyde was used as starting material.[2] The acetalization of the aldehyde group was carried out by standard methods[3] using diethoxymethoxyethane and ethanol to give 1-bromo-4-(diethoxymethyl)benzene. The formation of the Grignard compound with magnesium requires 1,2-dibromoethane and activation with ultrasound. Reaction with tri-n-butyl borate leads to the protected aryl boronic ester which gives after acidic work-up the target product in 78% yield.

The same reactants are forming with the aryl boronic ester at -60 °C 4-formylyphenylboronic acid with a 99% yield when activated with sodium bis(2-methoxyethoxy)aluminiumhydride, also on the kilogram scale.[4]
When the aryllithium compound of 1-bromo-4-(diethoxymethyl)benzene is used with triisopropylborate at -78 °C instead of the Grignard compound (via n-butyllithium) 4-formylphenylboronic acid is obtained in 99% crude yield.[5]

Disadvantages of both routes are the high price of the educts used (such as 4-bromobenzaldehyde, boronic esters of higher alcohols and butyllithium) as well as in the Nöth route the difficult workup after the hydrolysis by n-butanol. More recently, an improved process has been patented using less expensive starting materials such as 4-chlorobenzaldehyde, metallic lithium and trimethyl borate.[6]

4-Formylphenylboronic acid can also be prepared by hydrolysis of potassium 4-formylphenyl-trifluoroborate by means of acidic alumina[7] or silicon dioxide.[8] As a rule, phenylboronic acids serve as starting compounds for the corresponding potassium aryl trifluoroborates.[9]
Properties[edit]
4-Formylphenyl boronic acid crystallizes in colorless needles[2] or is obtained as an odorless, whitish powder, which dissolves little in cold but better in hot water. The compound is quite stable[4] and readily forms dimers and cyclic trimeric anhydrides, which complicate purification and tend to protodeboronize, a secondary reaction that occurs frequently in the Suzuki coupling, with elimination of the boronic acid function.[10]
Applications[edit]
4-Formylphenylboronic acid is used in Suzuki couplings, for example in the build up of pharmacologically active biphenyl compounds such as a precursor of the antihypertensive AT1 antagonist telmisartan in an improved synthesis:[11]
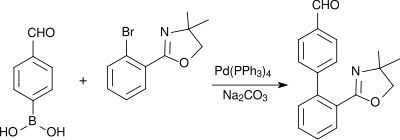
Also palladium-catalyzed aryl heteroaryl linkages after Suzuki use 4-formylphenylboronic acid as a molecular building block, as for instance in the synthesis of aryl-benzimidazole derivatives (which bind to peroxisome-proliferator-activated receptors (PPARγ) and activate the expression of a variety of genes):[citation needed]

In a copper-mediated fluoroalkylation reaction, the boronic acid group of the 4-FPBA can be replaced with perfluorinated alkyl iodides (Rf-I) by a perfluoroalkyl chain under mild conditions.[12]

4-Formyphenylboronic acid is used industrially as an enzyme stabilizer for proteases and in particular for lipases in liquid detergent preparations.[1] The addition of 4-FPBA in amounts < 0.08 wt% in the formulation reduces the loss of hydrolytic activity of the enzymes used and increases the storage stability of enzyme-containing liquid detergents.[13]
References[edit]
- ^ a b US 5972873, L.K. Nielsen, A. Deane-Wray, "4-Substituted-phenyl-boronic acids as enzyme stabilizers", published 1999-10-26, assigned to Novo Nordisk A/S
- ^ a b H. Feulner; G. Linti; H. Nöth (1990), "Beiträge zur Chemie des Bors, 206. Darstellung und strukturelle Charakterisierung der p-Formylbenzolboronsäure", Chem. Ber. (in German), 123 (9): 1841–1843, doi:10.1002/cber.19901230915
- ^ Autorenkollektiv, Organikum, 24. Auflage, S. 481, Wiley-VCH, Weinheim, 2001, ISBN 978-3-527-33968-6
- ^ a b H. Jendralla; A. Wagner; M. Mollath; J. Wunner (1995), "Efficient, simple procedures for the large-scale preparation of buildings blocks for angiotensin (II) receptor antagonists", Liebigs Ann. Chem., 1995 (7): 1253–1257, doi:10.1002/jlac.1995199507166
- ^ Y. Kobayashi; Y. Tokoro; K. Watatani (1998), "Preparation of functionalized zinc borates and their coupling reactions with allylic acetates", Tetrahedron Lett., 39 (41): 7537–7540, doi:10.1016/S0040-4039(98)01639-6
- ^ US 6833470, A. Meudt, S. Scherer, F. Vollmüller, M. Erbes, "Method for producing formylphenylboronic acids", published 2004-12-21, assigned to Clariant GmbH
- ^ G.W. Kabalka; V. Coltuclu (2009), "Thermal and microwave hydrolysis of organotrifluoroborates mediated by alumina", Tetrahedron Lett., 50 (46): 6271–6272, doi:10.1016/j.tetlet.2009.09.008
- ^ G.A. Molander; L.N. Cavalcanti; B. Canturk; P.-S. Pan; L.E. Kennedy (2009), "Efficient hydrolysis of organotrifluoroborates via silicagel and water", J. Org. Chem., 74 (19): 7364–7369, doi:10.1021/jo901441u, PMC 2763364, PMID 19743828
- ^ E. Vedejs; R.W. Chapman; S.C. Fields; S. Lin; M.R. Schimpf (1995), "Conversion of arylboronic acids into potassium aryltrifluoroborates: convenient precursors of arylboron difluoride Lewis acids", J. Org. Chem., 60 (10): 3020–3027, doi:10.1021/jo00115a016
- ^ G.K. Surya Prakash; F. Pertusati; G.A. Olah (2011), "HF-free, direct synthesis of tetrabutylammonium trifluoroborates", Synthesis, 2011 (2): 292–302, doi:10.1055/s-0030-1258370
- ^ A. S. Kumar; S. Ghosh; G.N. Mehta (2010), "Efficient and improved synthesis of Telmisartan", Beilstein J. Org. Chem., 25: 6, doi:10.3762/bjoc.6.25, PMC 2874342, PMID 20502601
- ^ Q. Qi; Q. Shen; L. Lu (2012), "Copper-mediated aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides at room temperature", J. Am. Chem. Soc., 134 (15): 6548–6551, doi:10.1021/ja301705z, PMID 22458339
- ^ US 20130252315, T. O’Connell, S. Tondera, T. Weber, "Stabilized, liquid, enzyme-containing surfactant preparation", published 2013-9-26, assigned to Henkel AG & Co. KGaA
