Hyrtioreticulin
| Names | |
|---|---|
IUPAC name
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
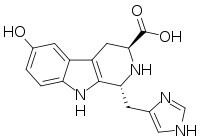
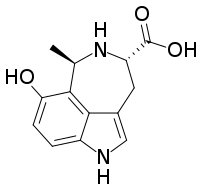
The hyrtioreticulins are a series of indole alkaloids, named hyrtioreticulin A through hyrtioreticulin F, that were isolated from Hyrtios reticulatus, an ocean sponge.[1][2]
Chemical structures[edit]
hyrtioreticulin A | hyrtioreticulin B | hyrtioreticulin C |
hyrtioreticulin D | hyrtioreticulin E | hyrtioreticulin F |
References[edit]
- ^ Yamanokuchi, Rumi; Imada, Kumiko; Miyazaki, Mitsue; Kato, Hikaru; Watanabe, Tadashi; Fujimuro, Masahiro; Saeki, Yasushi; Yoshinaga, Sosuke; Terasawa, Hiroaki; Iwasaki, Noriyuki; Rotinsulu, Henki (2012-07-15). "Hyrtioreticulins A–E, indole alkaloids inhibiting the ubiquitin-activating enzyme, from the marine sponge Hyrtios reticulatus". Bioorganic & Medicinal Chemistry. 20 (14): 4437–4442. doi:10.1016/j.bmc.2012.05.044. ISSN 0968-0896. PMID 22695182.
- ^ Imada, Kumiko; Sakai, Eriko; Kato, Hikaru; Kawabata, Tetsuro; Yoshinaga, Sosuke; Nehira, Tatsuo; Terasawa, Hiroaki; Tsukamoto, Sachiko (2013). "Reticulatins A and B and hyrtioreticulin F from the marine sponge Hyrtios reticulatus". Tetrahedron. 69 (34): 7051–7055. doi:10.1016/j.tet.2013.06.043.