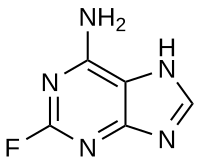
2-Fluoroadenine
| |
| Names | |
|---|---|
| IUPAC name
2-Fluoro-7H-purin-6-amine
| |
| Other names
2-FA
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.152.774 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H4FN5 | |
| Molar mass | 153.120 g·mol−1 |
| < 0.5 mM[1] | |
| Solubility | >=10 mM in DMSO |
| Hazards | |
| GHS labelling:[2] | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-Fluoroadenine (2-FA) is a toxic adenine antimetabolite which can be used in laboratory biological research for counterselection of wildtype bacterial[3] or eukaryotic (i.e. animals,[4] yeast,[5] plants,[6] diatoms,[7] brown algae[8]) APT (adenine phosphoribosyltransferase) genes. Therefore, knockouts or mutants for APT, which are resistant to 2-FA, can be selected.
2-Fluoroadenine is a critical intermediate for pharmaceutical drugs and can be synthesized within the lab from 2,6-diaminopurine, which is an inexpensive and readily available compound.[9] In the cell, 2-Fluoroadenine is synthesized and exhibits a large range of antibacterial activity. 2-Fluoroadenine acts as an inhibitor of blood-platelet adhesion, and when combined with actinobolin, produces a greater combined effect of preventing or treating infections.[10] In cancer treatments, 2-Fluoroadenine, has been used to treat head and neck cell carcinoma by the progressive removal of RNA and protein synthesis within tumor cells.[11]
See also[edit]
- 5-Fluoroorotic acid (5-FOA)
References[edit]
- ^ Kayushin, Alexey L.; Tokunova, Julia A.; Fateev, Ilja V.; Arnautova, Alexandra O.; Berzina, Maria Ya.; Paramonov, Alexander S.; Lutonina, Olga I.; Dorofeeva, Elena V.; Antonov, Konstantin V.; Esipov, Roman S.; Mikhailopulo, Igor A.; Miroshnikov, Anatoly I.; Konstantinova, Irina D. (2021-04-07). "Radical Dehalogenation and Purine Nucleoside Phosphorylase E. coli: How Does an Admixture of 2′,3′-Anhydroinosine Hinder 2-fluoro-cordycepin Synthesis". Biomolecules. 11 (4). MDPI AG: 539. doi:10.3390/biom11040539. ISSN 2218-273X. PMC 8067715. PMID 33917025.
- ^ "2-Fluoroadenine". pubchem.ncbi.nlm.nih.gov. Retrieved 4 May 2022.
- ^ Riley, Lauren A.; Guss, Adam M. (2021-01-25). "Approaches to genetic tool development for rapid domestication of non-model microorganisms". Biotechnology for Biofuels. 14 (1). Springer Science and Business Media LLC: 30. doi:10.1186/s13068-020-01872-z. ISSN 1754-6834. PMC 7830746. PMID 33494801.
- ^ Schaff, D A; Jarrett, R A; Dlouhy, S R; Ponniah, S; Stockelman, M; Stambrook, P J; Tischfield, J A (1990). "Mouse transgenes in human cells detect specific base substitutions". Proceedings of the National Academy of Sciences of the United States of America. 87 (21): 8675–8679. Bibcode:1990PNAS...87.8675S. doi:10.1073/pnas.87.21.8675. PMC 55020. PMID 2236079.
- ^ Sahota, Amrik; Ranjekar, Prabhakar K.; Alfonzo, Juan; Lewin, Alfred S.; Taylor, Milton W. (1987). "Mutants of Saccharomyces cerevisiae deficient in adenine phosphoribosyltransferase". Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 180 (1). Elsevier BV: 81–87. doi:10.1016/0027-5107(87)90069-8. ISSN 0027-5107. PMID 3306356.
- ^ Schaff, Dennis A. (1994). "The adenine phosphoribosyltransferase (APRT) selectable marker system". Plant Science. 101 (1). Elsevier BV: 3–9. doi:10.1016/0168-9452(94)90159-7. ISSN 0168-9452.
- ^ Serif, Manuel; Dubois, Gwendoline; Finoux, Anne-Laure; Teste, Marie-Ange; Jallet, Denis; Daboussi, Fayza (2018-09-25). "One-step generation of multiple gene knock-outs in the diatom Phaeodactylum tricornutum by DNA-free genome editing". Nature Communications. 9 (1). Springer Science and Business Media LLC: 3924. Bibcode:2018NatCo...9.3924S. doi:10.1038/s41467-018-06378-9. ISSN 2041-1723. PMC 6156588. PMID 30254261.
- ^ Badis, Yacine; Scornet, Delphine; Harada, Minori; Caillard, Céline; Godfroy, Olivier; Raphalen, Morgane; Gachon, Claire M. M.; Coelho, Susana M.; Motomura, Taizo; Nagasato, Chikako; Cock, J. Mark (2021-07-10). "Targeted CRISPR-Cas9-based gene knockouts in the model brown alga Ectocarpus" (PDF). New Phytologist. 231 (5). Wiley: 2077–2091. doi:10.1111/nph.17525. ISSN 0028-646X. PMID 34076889. S2CID 235295486.
- ^ Salehi Marzijarani, Nastaran; Snead, David R.; McMullen, Jonathan P.; Lévesque, François; Weisel, Mark; Varsolona, Richard J.; Lam, Yu-hong; Liu, Zhijian; Naber, John R. (2019-08-16). "One-Step Synthesis of 2-Fluoroadenine Using Hydrogen Fluoride Pyridine in a Continuous Flow Operation". Organic Process Research & Development. 23 (8): 1522–1528. doi:10.1021/acs.oprd.9b00178. ISSN 1083-6160.
- ^ Montgomery, John; Hewson, Kathleen (May 1969). "Nucleosides of 2-Fluoroadenine". Journal of Medicinal Chemistry. 12 (3): 498–504. doi:10.1021/jm00303a605. ISSN 0022-2623.
- ^ Behbahani, Turang E.; Rosenthal, Eben L.; Parker, William B.; Sorscher, Eric J. (June 2019). "Intratumoral generation of 2-fluoroadenine to treat solid malignancies of the head and neck". Head & Neck. 41 (6): 1979–1983. doi:10.1002/hed.25627. ISSN 1097-0347. PMC 6531318. PMID 30633420.
